Write a Molecular Formula for This Molecule
NH2 Write out the i structural formula ii Lewis structure iii condensed formula iv. C 4 H 6.

Clo3 Polar Or Non Polar Chlorate Ion Polar Molecules Pill
Pick an element to list first in the formula and add a subscript.

. Identify the element and the count of atoms based on the color coded molecular model. In this system the correct molecular formula format is. Write the molecular formula in a linear form for example X 2 Y 5 Z 11.
The key is memorizing the prefixes. HO-CHCH-OH HOCCOH CI CI CH3 CICC CH3 CH3 CCH-CH3 none of the above Draw the skeletal line structure of a cyclic ketone with 5 carbons in the ring and 1 methyl group. When carbon and oxygen combine to form carbon dioxide we represent it as.
Many of the prefixes youll be familiar with since they are part of. Write the molecular and empirical formulas of the following compounds. The molecular formula indicates the exact number of atoms in the molecule.
The molar mass was given as 60 gmol. Oxygen 19419 x 01648. A What is the molecular formula of the molecule below.
The molecular formula for glucose is C 6 H 12 O 6. If you are given percent composition you can. Typically non-metals tend to share electrons make covalent bonds.
Write a skeleton molecular formula using the symbols for carbon C hydrogen H and oxygen O. Separate the chemical symbols for each element in the molecular formula by substituting the word and for. Molecular compounds are made when two or more elements share electrons in a covalent bond to connect the elements.
Its okay to look up the atomic. 1 molecule 2 molecules 1 molecule 2 molecules 6022x10 23 molecules 2x6022x1023 molecules 6022x10 molecules 2x6022x1023 molecules 1 mole 2 moles 1 mole 2. When a molecule has bonding other than covalent groups of atoms such.
Illustrative Example Lets say a gaseous compound consists of 7026 carbon 953 oxygen and 2021 hydrogen. Then we should find out how much CH 2 O units are present there. For that first calculate the molar mass of empirical formula and then.
The empirical formula expresses the smallest whole number. Write the molecular formula for the molecule. Formula writing for molecular compounds is probably the easiest type of formula writing.
OH HN b For the following molecule. Write the molecular formula for the following molecule. How to determine the molecular formula of a water molecule bearing molar mass of 20gmol.
HC 2 H 3 O 2. For example if the molecule has 12 carbons 24 hydrogens and one oxygen enter C12H60. Carbon hydrogen then all other elements in alphabetical order.
For example carbon has an atomic weight of 120107 hydrogen has an atomic weight of 100794 and oxygen has an atomic weight of 159994. I see three grey balls in. Multiply percent composition with the molecular weight.
C 2 H 3. Determine the mass in grams of each element in the sample. OH Use the format CHO.
C 4 H 8. Carbon 19419 x 04948 960852. H 2 CO 3.
For example the molecule acetylene has molecular formula C 2 H 2 but the. Each element has a symbol. Hydrogen 19419 x 00519 1007846.
CO 2 is the molecular formula. TextEmpirical Formula Mass 2100815999 textEmpirical Formula. The chemical formulae for all the elements that form each molecule and.
For instance C is symbol of carbon and O is that of oxygen. Theres a particular way of writing whats in a molecule called a chemical formula. A b c d Answer a.
Formula Anion Name Acid Name. You start by determining the empirical formula for the compound.

How Do You Write Chemical Formula For An Ionic Compound Wedding Jewellery Inspiration Delicate Jewelry Necklace Antique Jewelry Necklace
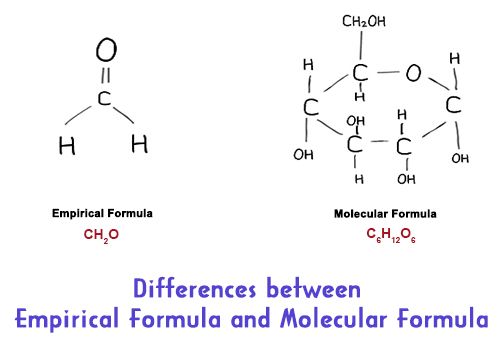
Differences Between Empirical Formula And Molecular Formula Qs Study

Pin By Qkmaqcba On Communications Logos Negative Space In 2022 Molecule Model Communication Logo Chemical Formula

Comments
Post a Comment